Pharmaceutical companies face unique challenges in a constantly changing and increasingly volatile environment.
It is becoming both more important and more complex to efficiently ensure market access for innovative therapies at a sustainable price. Once this step has been mastered, the product must be optimally supported throughout its lifecycle. It is important not only to anticipate and support any extensions of indications and regular price reviews but also to strategically plan and implement the change steps necessary for timely product support. The disruption associated with the expiry of protection periods can be actively responded to with individually adapted strategies.
HEMAP supports you in critical phases of the product lifecycle. Our competencies are based on decades of successful work in marketing, market access, pricing, lifecycle management, change management, business development and external and public affairs at leading pharmaceutical companies in Switzerland and at regional/global level.
Our core competencies:
Our partner in Austria:
Our US partner:
We cooperate with:
MARKET ACCESS
«Market access is everything – and everything comes to nothing without market access.»
Based on our many years of experience and networking, we can offer you support with the following challenges:
- Development of an access and pricing strategy for new products and indications
- Optimization of cost-benefit arguments
- Medical-economic analyses (e.g. influence of new treatment on resource consumption in the healthcare system)
- Support for price reviews or new applications for time-limited products
- Preparation of the necessary dossiers and applications
- Support in negotiations with authorities
- Second opinion on strategies developed in-house
- Support in the event of vacancies or capacity bottlenecks
Process analysis
New, innovative forms of therapy are changing the way patients are treated, now and in the future. For example, the hospital stay is shortened and/or the treatment involves other services or requires other resources, which in turn has a significant impact on the overall costs.
The process analysis records the individual steps of the treatment in detail (e.g. personnel requirements, laboratory, treatments, length of stay, etc.) along with both the costs incurred and the costs charged. This makes it possible to show the impact of a new type of treatment on the costs and revenues of service providers.
Using a concrete example, it was possible to show that a novel drug for the treatment of a hematological disease in hospital has the following effects:
- the time required to treat a patient with the new therapy are reduced by around a third (-31%),
- the greatest reduction was in nursing time (-36%), a critical resource due to the shortage of specialist staff,
- additional diagnostic resources need to be provided.
(Ref. Ring A, Grob B, Aerts E, Ritter K, Volbracht J, Schär B, Greiling M, Müller AMS. Resource utilization for chimeric antigen receptor T cell therapy versus autologous hematopoietic cell transplantation in patients with B cell lymphoma. Ann Hematol. 2022 Aug;101(8):1755-1767. doi: 10.1007/s00277-022-04881-0)
This type of analysis
- opens up new holistic possibilities in assessing the impact of new therapies beyond the purely material cost perspective,
- leads to more informed decision-making in terms of the overall impact on service providers,
- additionally shows which changed challenges new forms of therapy pose to the resource planning and organization of health care facilities, and
- helps to ensure that the market launch of new forms of therapy is not delayed by organizational obstacles.
Software-supported analysis
To minimize the time spent by healthcare professionals, the process analysis is supported, documented, and evaluated by propriety software developed with our Austrian partner company AXXESS Healthcare Consulting. The software contains already hundreds of services and process steps. Therapy plans can be co-created with experts by simple drag & drop of elements within a day or between individual days.
You can find more detailed information at:
Life-cycle eXcellence
The following 4 transitions in the life cycle of a product are crucial for success:
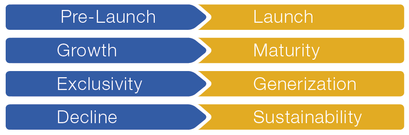
We support you in these critical phases with our many years of experience in market access and international marketing.
A) Pre-launch à launch
- How can we achieve a product profile that is still competitive and offers significant clinical added value even after a long development period?
- How can a marketing authorization (indication, specialist information) be achieved that meets the medical need, makes clinical sense and creates a good starting position for reimbursement and pricing?
- How can reimbursement be achieved at a sustainable price that ensures broad access for patients?
- How do you achieve a change in employees’ focus from internal issues in the admission phase to an external customer focus?
B) Growth à maturity
- How can the price development be optimized via reviews and possible extensions of indication?
- How can the product be optimally supported and the ROI optimized even with declining growth?
- How can the product quality level be maintained and extended?
C) Exclusivity à generization
- What is the optimal strategy in terms of sales/marketing and price? An early analysis can be decisive here.
- How do you achieve change for employees in order to motivate them to tackle the new challenges?
- What does a competitive price and discount strategy look like?
D) Decline à sustainability
- What is the optimal pricing and portfolio strategy?
- How can support from a legacy portfolio be designed to optimize ROI?
- What needs to be considered when hiring or selling products?

Frank Weber LINKEDIN
CEO & Founder HEMAP AG
MSc ETH, PhD
30 years experience in the pharmaceutical industry in sales, marketing, general management, market access, external & public affairs.
Excellent network in the healthcare sector, among others with authorities, associations and stakeholders.
Partner HEMAP AG
MSc UZH
20 years experience in the pharmaceutical industry in sales, marketing, life cycle management, business development and change management.
National and international career and network.
Partner HEMAP AG
25 years of experience in the pharmaceutical industry and the medtech sector in the areas of general management, country lead, sales & key account management, engineering, marketing and software-based procedural health economic analyzes in the healthcare sector.Dammstrasse 19
6300 Zug
Switzerland
+41 79 431 59 53
+41 76 499 58 28





